The electron configuration can be determined from where the atom. For this example we will use the iodine atom.
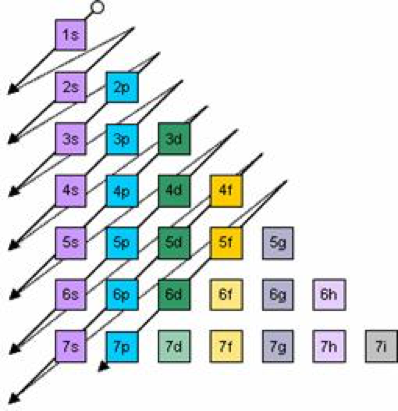
Electron Configurations Orbitals Energy Levels And Ionisation Energy Trends A Level Chemistry Revision Notes
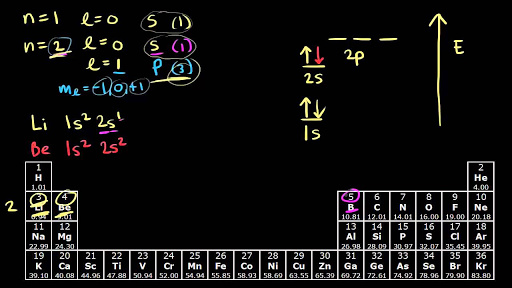
Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital.
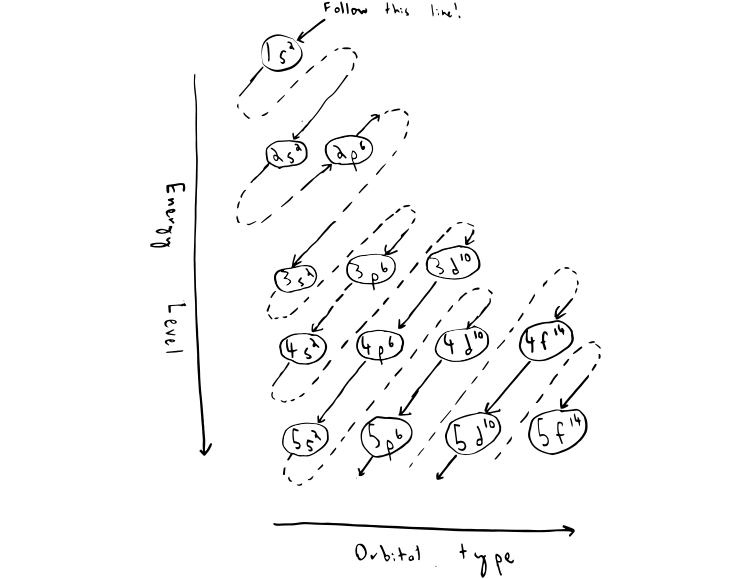
Electron configuration level two.
The electron configuration indicates that bromine has a total of 35 electrons.
Because the second energy level 2s 2 2p 6 has eight electrons Neon has an octet and has a full outer shell.
In writing the electron configuration for Argon the first two electrons will go in the 1s orbital.
The remaining six electrons will go in the 2p orbital.
By convention we therefore write abbreviated electron configurations in terms of the number of electrons beyond the previous element with a filled-shell electron configuration.
An atoms electron configuration describes the way its electrons fill sublevels when the atom is in its ground state.
The attraction between this lone valence electron and the nucleus with 11 protons is shielded by the other 10 core electrons.
Electron configurations of the next two elements in the periodic table for example could be written as follows.
When we write the configuration well put all 18 electrons in orbitals around the nucleus of the Argon atom.
6 is 1s 2 2s 2 2p 2.
For a main-group element the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n.
However notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for Argon a noble gas.
The electron configuration of bromine is 1s2 2s2p6 3s2p6d10 4s2p5 which can be shortened to Ar 4s2 3d10 4p5.
Just replace this portion of zincs electron notation with Argons chemical symbol in brackets Ar So zincs electron configuration written in shorthand is Ar4s 2 3d 10.
Mg Z 12.
Periodic Table Exceptions To Know.
For example the electron configuration of the neon atom is 1s2 2s2 2p6 using the notation explained below.
Therefore the Ne electron configuration will be 1s 2 2s 2 2p 6.
The electrons that determine valence how an atom reacts chemically are those with the highest energy.
There is a surface between the two balls where there is zero probability of finding an electron.
For example the electronic configuration of carbon atomic number.
Na Z 11.
Not all electrons inhabit s orbitals.
It carries a negative charge of 1602176634 10 19 coulomb which is considered the basic unit of electric chargeThe rest mass of the electron is 91093837015 10 31 kg which is only 1 1836 the mass of a protonAn electron is therefore considered nearly massless in comparison with a proton or a neutron and the electron mass is.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10.
Electronic Structure Test Questions.
Thus the number of valence electrons that it may have depends on the electron configuration in a.
In these cases a completely full or half full d sub-level is more stable than a partially filled d sub-level so an electron from the 4s orbital is excited and rises to a 3d orbital.
There are two main exceptions to electron configuration.
Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.
At the first energy level the only orbital available to electrons is the 1s orbital.
H 1s1 He 1s2 Li 1s2 2s1 Be 1s2 2s2 B 1s2 2s2 2p1 C 1s2 2s2 2p2 N 1s2 2s2 2p3 O 1s2 2s2 2p4 F 1s2 2s2 2p5.
The outer energy level is n 3 and there is one valence electron.
An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons may occupy.
Zincs full electron configuration is.
Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital.
A bromine atom has two electrons in its first energy level eight electrons in its second 18 electrons in its third and seven electrons in its fourth.
Electron lightest stable subatomic particle known.
There are two ways in which electron configuration can be written.
The electron configuration for the first 10 elements.
There is a major exception to the normal order of electron configuration at Cr 24 and Cu 29.
The next six electrons will go in the 2p orbital.
The s sublevel can only hold two electrons so the 1s is filled at helium 1s 2.
Youll be able to see how many electrons occupy the highest energy level.
The electron configuration for cesium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6.
We call this surface a node or a nodal surface.
The two elements are indicated on the periodic table.
The element sodium has the electron configuration 1s 2 2s 2 2p 6 3s 1.
The electrons occupying the orbitals of varying energy levels.
Principal Energy Level Definition.
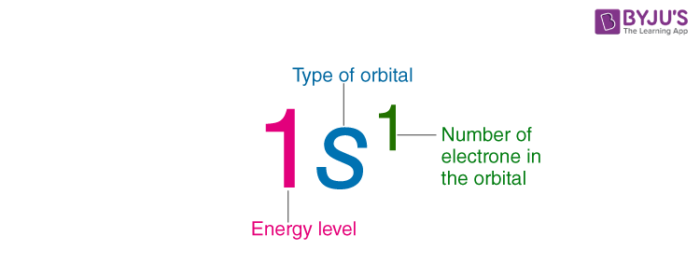
While writing electron configurations a standardized notation is followed in which the energy level and the type of orbital are written first followed by the number of electrons present in the orbital written in superscript.
It turns out that the energy the electron configuration that is half-filled 4s 1 3d 5 and filled orbital 4s 1 3d 10 has lower energy than the typical filling order 4s 2 3d 4 and 4s 2 3d 9This pattern is followed in the 5 th row with Mo 42 and Ag 47.
To help describe the appropriate notation for electron configuration it is best to do so through example.
According to the Aufbau principle the electrons of an atom occupy quantum levels or orbitals starting from the lowest energy level and proceeding to the highest with each orbital holding a maximum of two paired electrons opposite spins.
A 3s orbital is even larger and it has three nodes.
N atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals.
It is therefore a Nobel Gas.
The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6.
Electron shell 1 has the lowest energy and its s.

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes






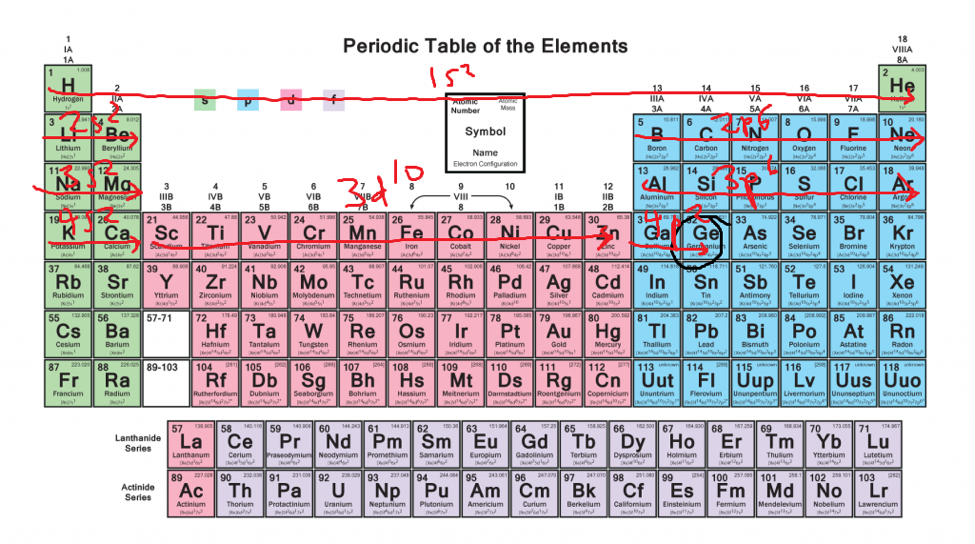










No comments:
Post a Comment