It is these dd transitions ligand to metal charge transfers LMCT or metal to ligand charge transfers MLCT that generally give metals complexes. The third electron goes into the next orbital in the energy diagram the 2s orbital.

Chemistry291 Hand Note Electronic Configuration For Chlorine Cl In Just Electron Configuration Chemistry Help Chemistry Notes
The full version of bismuths electron configuration.

Electron orbital diagram.
Combining a pair of helium atoms with 1s 2 electron configurations would produce a molecule with a pair of electrons in both the bonding and the antibonding molecular orbitals.
Such a region of space is called an orbital.
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of.
The boxes are used to represent the.
The area where an electron is most likely to be found is called its orbital.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f.
There is a major exception to the normal order of electron configuration at Cr 24 and Cu 29.
Because this orbital is so small and retains its electrons so tightly it does not contribute to bonding.
1s 2 2s 2.
This gives us an orbital filling diagram.
Likewise the orbital correlation diagram for methane provides another example of the difference in electron density predicted by molecular orbital calculations from that of the localized bond model.
The lobes of a p orbital disappear at the nucleus.
Li Z 3.
We need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO molecular orbital method in particular.
You can think of an orbital as being the region of space in which the electron lives.
The fourth electron fills this orbital.
The probability of finding an electron at the nucleus is 0 you will never find an electron in the nucleus.
Click on the compound names for.
Below is a diagram that shows the probability of finding an electron around the nucleus of a hydrogen atom.
There are lots of quizzes on electron configurations you can practice with located here.
Using the Molecular Orbital Model to Explain Why Some Molecules Do Not Exist.
After the 1s and 2s orbitals have been filled the next lowest energy orbitals are the three 2p orbitals.
I skipped past beryllium because I was getting bored.
The TanabeSugano diagram with a small amount of information accurately predicts absorptions in the UV and visible electromagnetic spectrum resulting from d to d orbital electron transitions.
Orbital Diagram For Nitrogen N Nitrogen Electron Configuration February 23 2021 February 15 2021 by Sneha Nitrogen Electron Configuration.
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
This molecular orbital model can be used to explain why He 2 molecules dont exist.
1s 2 2s 1.
The electron configuration of boron is 1s²2s²2p¹ which means that there are two electrons in the 1s orbital two electrons in the 2s orbital and one electron in the 2p orbitals.
This is why the hydrogen atom has an electron configuration of 1s 1.
This orbital is equivalent to the innermost electron shell of the Bohr model of the atom.
The 2px orbital lies on the x-axis.
The following diagram shows the orbitals that are filled when one goes across the periods.
The orbital filling diagram of boron.
It is called the 1s orbital because it is spherical around the nucleus.
When we talk about school subjects then one of the major subjects which are very important for knowledge perspective is science.
It turns out that the energy the electron configuration that is half-filled 4s 1 3d 5 and filled orbital 4s 1 3d 10 has lower energy than the typical filling order 4s 2 3d 4 and 4s 2 3d 9This pattern is followed in the 5 th row with Mo 42 and Ag 47.
For example the electron configuration of the neon atom is 1s2 2s2 2p6 using the notation explained below.
1 An orbital is a three dimensional description of the most likely location of an electron around an atom.
Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as the little boxes.
The total energy of an He 2 molecule would be.
95 of the time or any other percentage you choose the electron will be found within a fairly easily defined region of space quite close to the nucleus.
Thus by looking at the location of bismuth we create its electron configuratoin by adding all the different pieces that are added as we go down the periodic table.
In an orbital filling diagram the individual orbitals are shown as circles or squares and orbitals within a sublevel are drawn next to each other horizontally.
Be Z 4.
The diagram shows a cross-section through this spherical space.
Notice that the 1s orbital has the highest probability.
The 3py orbital lies on the y-axis and is larger than the 2px orbital.
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
Electronic configurations describe each electron as moving independently in an orbital in an average field created by all other orbitals.
The distribution of electrons among the orbitals of an atom is called the electron configurationThe electrons are filled in according to a scheme known as the Aufbau principle building-up which corresponds for the most part to increasing energy of the subshells.
The lithium 1s orbital is the lowest-energy orbital on the diagram.
If you guys have come across our recent article then it would be easy for you all to understand the concept.
The closest orbital to the nucleus called the 1s orbital can hold up to two electrons.
Here the correlation diagram correctly accounts for the paramagnetic character of this simple diatomic compound.
What does this tell us about electrons in p orbitals.
Orbital Diagram For Carbon C Carbon Electron Configuration February 23 2021 February 20 2021 by Sneha Carbon Electron Configuration.
Periodic Table Exceptions To Know.

Electron Configurations Using Orbital Diagrams N 3 And 4 Only Electron Configuration Learning Worksheets Electrons

Ground State Electron Configuration Google Search Electron Configuration Chemistry Worksheets Geometry Worksheets

29 Cu Copper Electron Shell Structure Schoolmykids Electron Configuration Element Symbols Periodic Table Of The Elements

Electron Configuration For Boron Spdf Electronic Configuration Chemistry Atomic Number 5 In 2021 Electron Configuration Chemistry Education Chemistry

Drawing Electron Configuration Diagrams Chemistry For All The Fuse School Electron Configuration Chemistry Protons

What Information Does The Electron Configuration Give Us About Elements Pick Out An Element From Each Chemistry Lessons Chemistry Classroom Teaching Chemistry

12 Mg Magnesium Electron Shell Structure Schoolmykids Electron Configuration Element Chemistry Magnesium

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

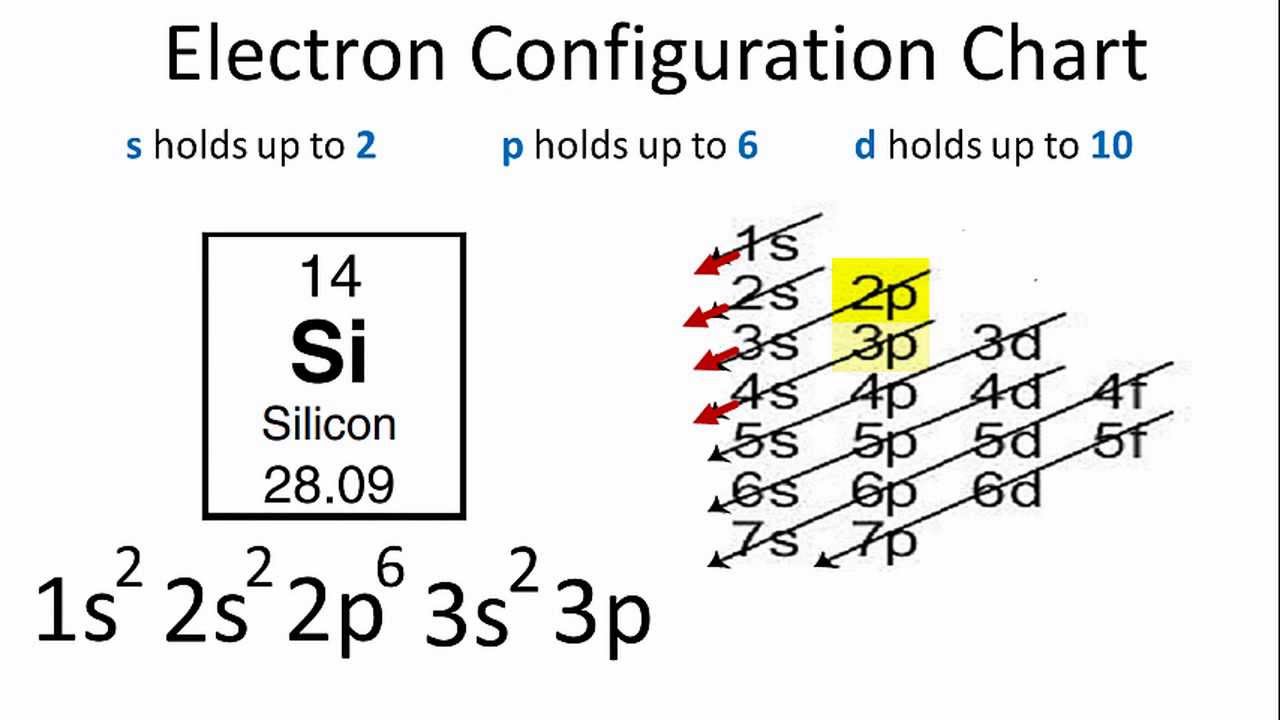
Easiest To Follow Electron Configuration Chart I Have Found Chemistry Lessons Chemistry Classroom Teaching Chemistry

Electron Configuration Tricks Ions Shortcuts Teaching Chemistry Chemistry Worksheets Electron Configuration

Electronic Configurations Chemwiki Electron Configuration Complex Sentences Worksheets Text Features Worksheet

Orbital Diagrams And Electron Configuration Worksheet Yahoo Image Search Results Electron Configuration Chemistry Worksheets Word Problem Worksheets

Chemistry291 Hand Note 5 Steps Electron Configuration For Potassium Or O In 2021 Electron Configuration Configuration Teaching Chemistry

Electron Configuration And Orbital Diagram Power Point W T Electron Configuration Chemistry Foldables Electrons

Diagram Of Orbital Energies With Illustrations Of S P And D Orbital Types Teaching Chemistry Chemistry Notes Organic Chemistry







No comments:
Post a Comment