
Chemical Properties 1 Electron Configuration Sulfur Is In Group 16 A Period 3 It Is In The Non Metal Section Of The Periodic Table And Holds A Level 3 Energy Level This Upper Right Section Of Non Metals Are The Most Reactive And Have The Highest

The Electronegativity Of Oxygen Is Greater Than Sulphur Whereas The Electron Affinity Of Sulphur Is Greater Than Oxygen Aren T These Statements Contradictory Quora

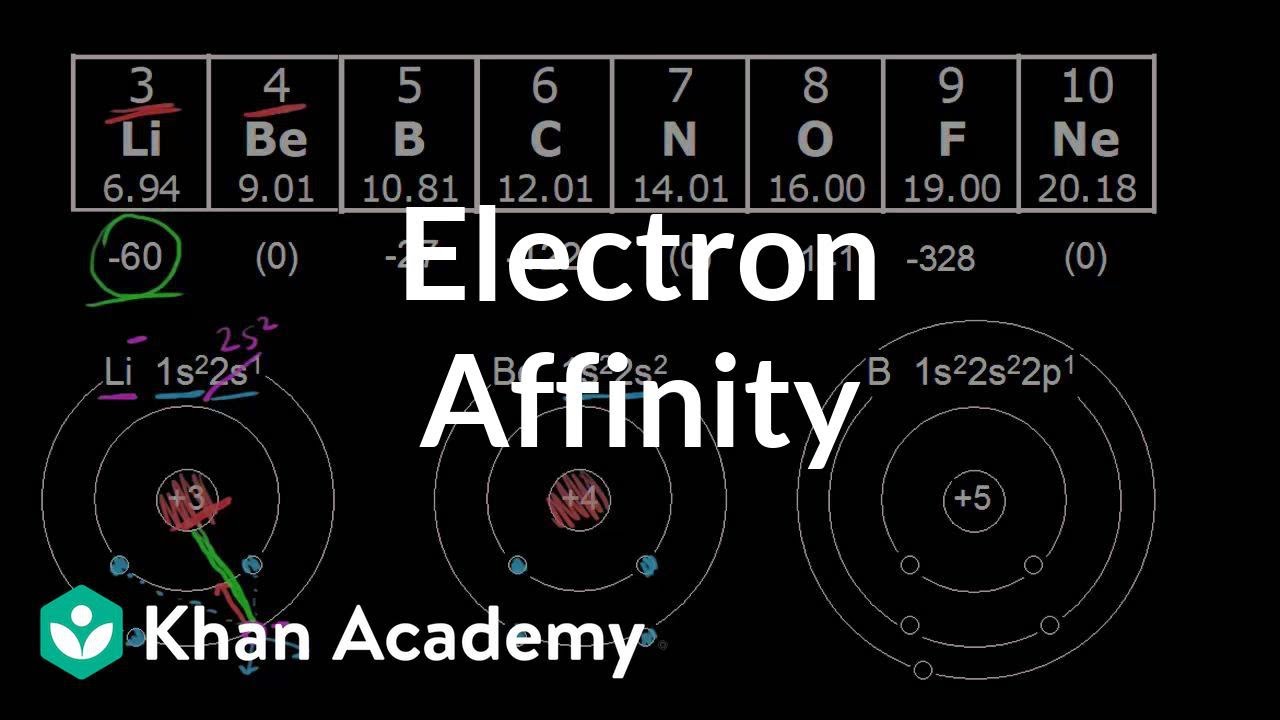
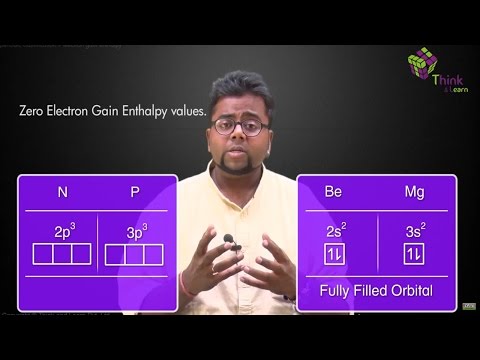
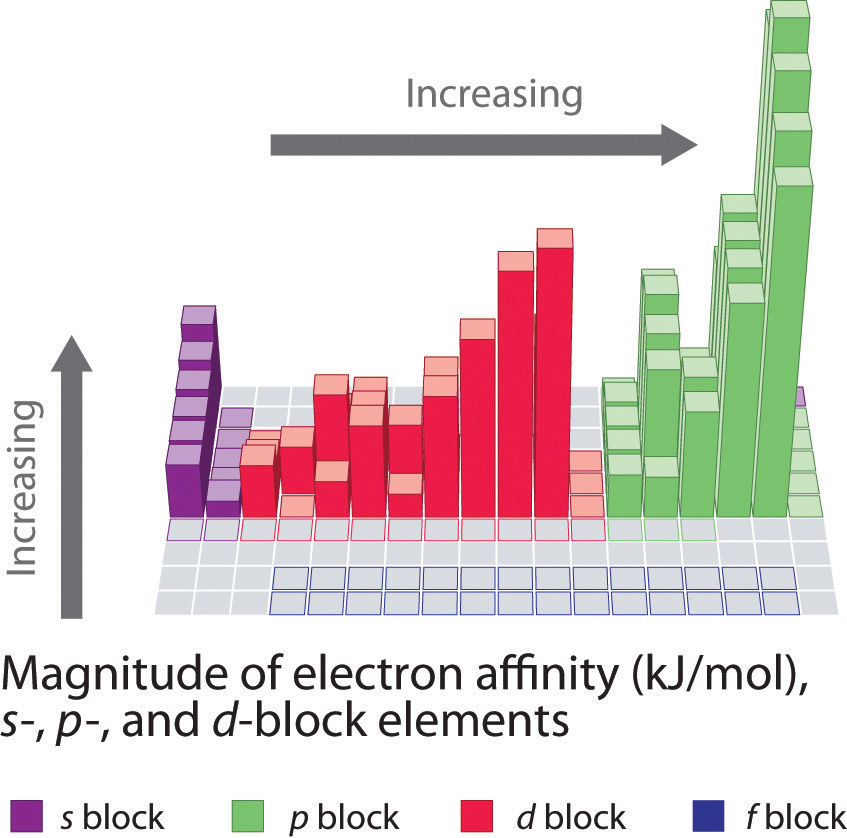
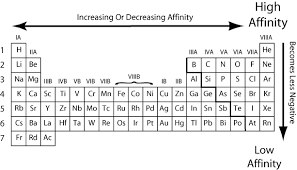
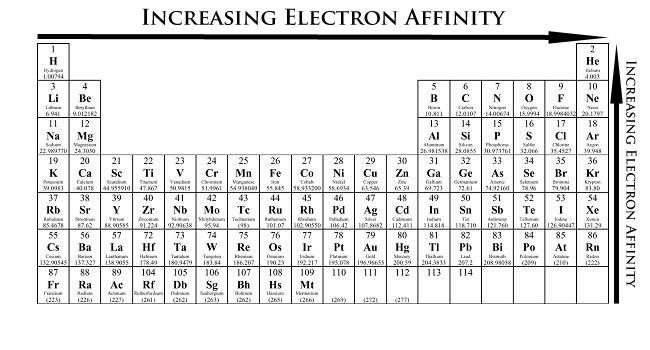
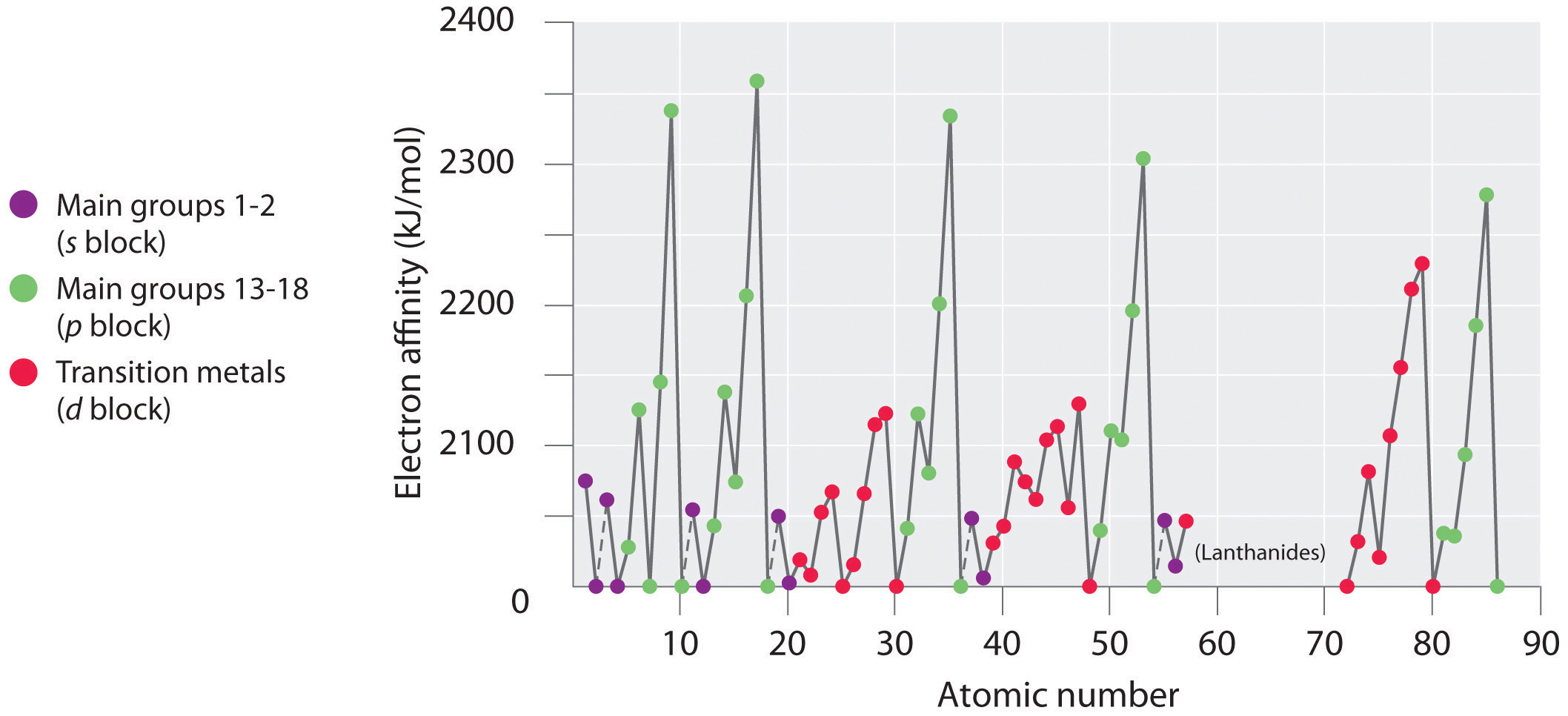
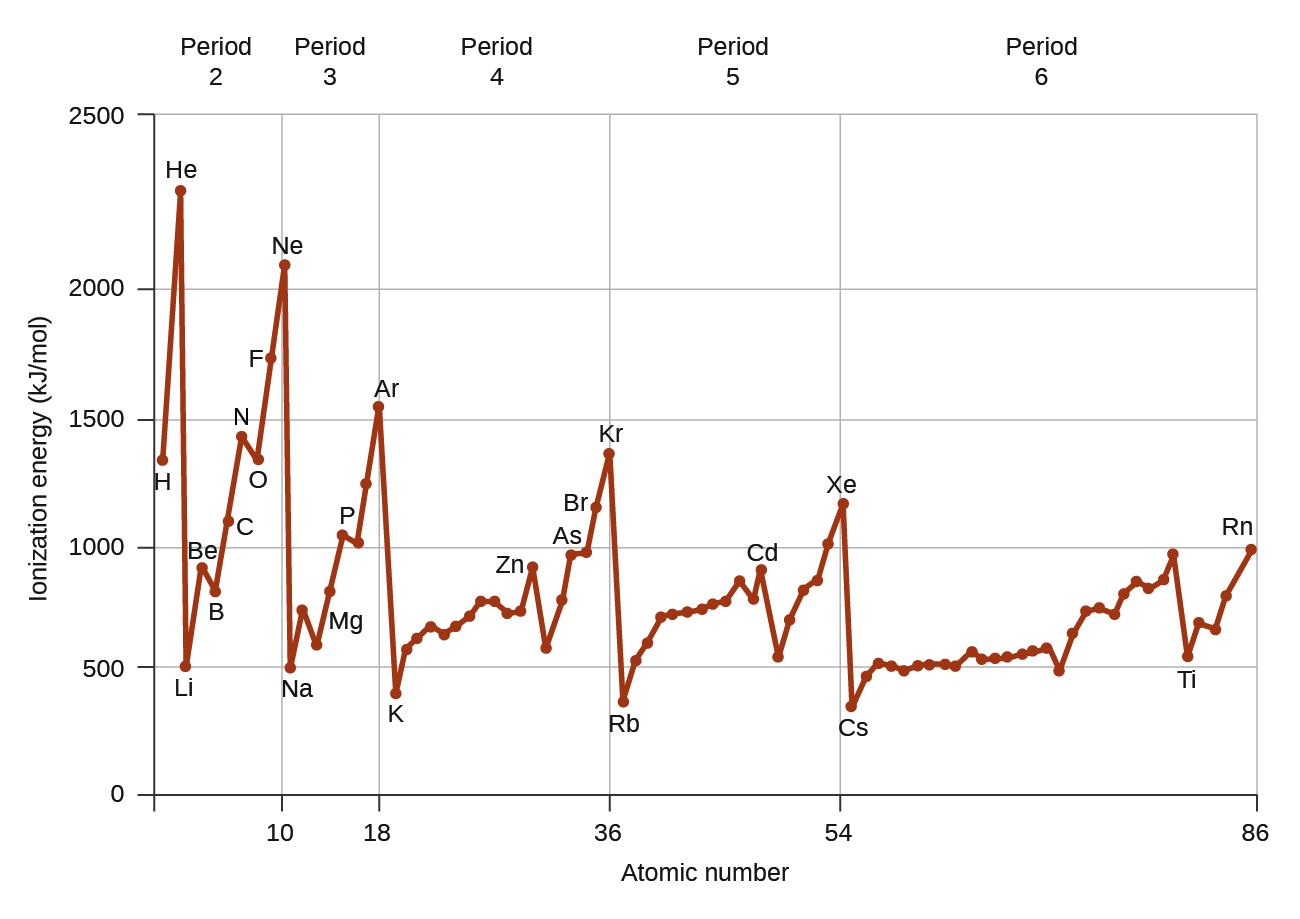
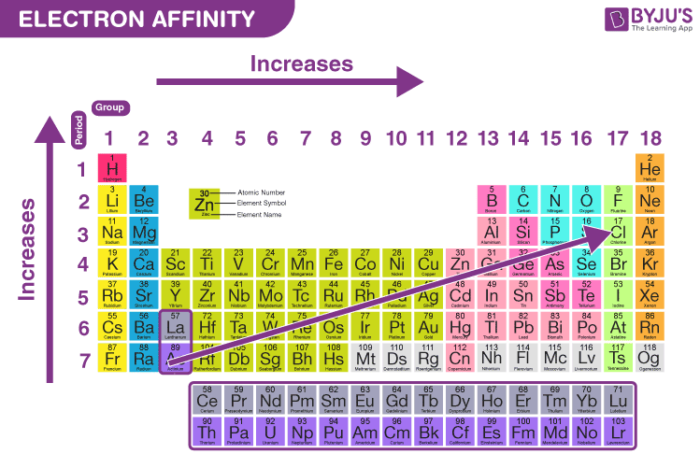
Why Does The Electron Affinity Increase Become More Exothermic Down Group 2 And Group 5 Chemistry Stack Exchange

What Happens To Electron Affinity Along The Period And Down The Group In The Periodic Table Why Quora







No comments:
Post a Comment