4s 2 4p 6 xenon Xe. Actinium Atomic Weight.

15 P Phosphorus Electron Shell Structure Schoolmykids Element Chemistry Shell Structure Periodic Table Of The Elements
This group of inert or noble gases also includes krypton Kr.

Electronic configuration of group 13 elements.
All of the classifications include the elements gallium indium tin thallium lead and bismuth.
For example TiZ 22 is in period 4 so that n 4 the first 18 electrons have the same configuration of Ar at the end of.
An electron configuration can quickly and simply tell a reader how many electron orbitals an atom has as well as the number of electrons populating each of its orbitals.
An atoms electron configuration is a numeric representation of its electron orbitals.
5s 2 5p 6 and radon Rn.
The lanthanum electronic configuration Xe4f 0 5d 1 6s 2 and lutetium electronic configuration Xe4f 14 5d 1 6s 2 have no partially filled 4f-orbital in their ground state are considered as lanthanides due to their properties close to these elements.
Electronic configuration of noble gases.
Beryllium 1s 2 2s 1.
Groups 1-2 termed s-block elements.
Characteristics of Group 18 Elements.
Post-transition metals are generally considered to be elements in Groups 13 14 and 15.
It has 5 valence electrons.
The general electronic configuration of the d-block elements is n 1d 110 ns 02Here noble gas is the configuration of the last noble gas preceding the atom in question and n is the highest principal quantum number of an occupied orbital in that atom.
The form of the periodic table is closely related to the electron configuration of the atoms of the elements.
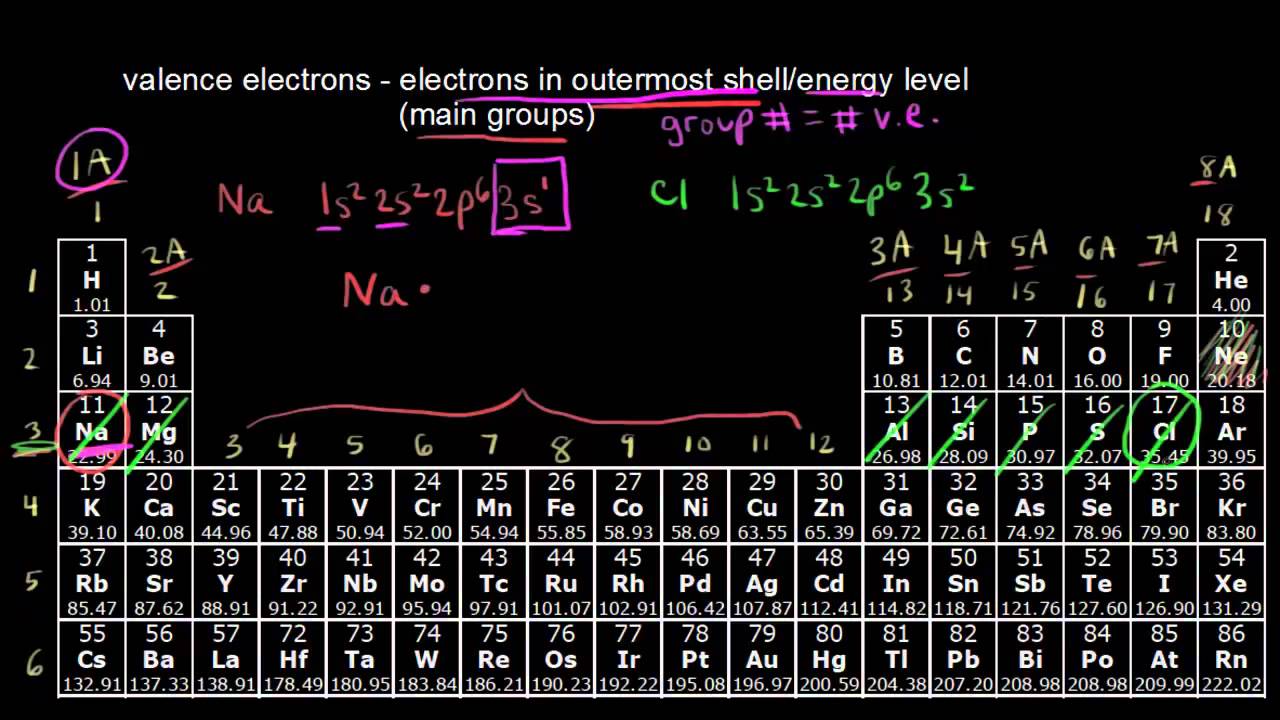
Na group 1 has one A1 group 13 has three 13 -10 Si group 14 has four 14-10 and P group 15 has five 15 10 valence electrons.
6s 2 6p 6.
Groups 3-11 are termed transition elements.
Group 3A or 13 all end their electron configurations in p1.
Carbon 1s 2 2s 2 2p x 1 2p y 1.
The valence shell electronic configuration of these electrons is ns 2 np 5.
In group 4A or 14 all elements end in p2.
In general the periodicity of the periodic table in terms of periodic table blocks is clearly due.
Electronic Configuration of Group 17 Elements.
Electronic Configuration of the d-Block Elements.
1111 Electronic Configuration The outer electronic configuration of these elements is ns2np1.
Transition Metals Electron Configuration.
Thus in the building-up process for the lanthanoids.
The other members of group 8 have a characteristic valence shell electron octet ns 2 np x 2 np y 2 np z 2.
In the periodic table above these elements are colored beige.
Groups 3-12 are termed d-block elements.
B B belongs to group 15 because its electronic configuration is 2 5 ie.
Boron ends in 2p1.
And so it goes.
Thus these elements look out to either lose one electron and form a covalent bond or.
A close look at the electronic configuration suggests that while boron and aluminium have noble gas core gallium and indium have noble gas plus 10 d-electrons and thallium has noble gas plus 14 f-electrons plus 10 d-electron cores.
A A belongs to group 13 because its electronic configuration is 2 3 ie.
The general electronic configuration of these elements is Xe4f 0 14 5d 0-1 6s 2.
The electronic configuration of an atom is the represents the arrangement of the electrons distributed among the shells and subshells.
The d-block elements may also be known as Transition Elements as they are elements which lie between the metals and non-metals of the periodic.
In order to make the electronic configuration of the elements simple and convenient it is written such that the electronic configuration of the noble gas core precedes the valence shell electrons.
The group number is an identifier used to describe the column of the standard periodic table in which the element appears.
The first example occurs in the case of the lanthanoids elements having atomic numbers between 57 and 71The lanthanoids have the general electron configuration Kr4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2.
Thus they exhibit a stable octet configuration.
Members of group 18 have eight valence electrons ie they have eight electrons in their outermost orbit except helium.
Thus there are 7 electrons in the outermost shell of these elements.
Where i is a number between 0 and 14.
Group 2 elements 2A the alkaline earth metals all end in s2.
Groups 1-2 except hydrogen and 13-18 are termed main group elements.
Atomic number 12 2 8 2 Atomic number 19 2 8 8 1 Atomic number 20 2 8 8 2.
For the transition metals groups 3-12 there are many exceptions.
In other words electronic configuration describes how the electrons are assembled in the shells and subshells of the atoms.
They belong to 2nd period as they both have two shells.
The electronic configuration of elements with.
2270 Atomic Number.
Ac Name of Element.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d1.
Therefore P has maximum number of valence electrons ie3.
The third major category of elements arises when the distinguishing electron occupies an f subshell.
Electron orbitals are differently-shaped regions around an atoms nucleus where electrons are mathematically likely to be located.
But helium exhibits a duplet configuration.
However depending on how post-transition is defined this category may contain as few as six or as many as 22 elements.
It has 3 valence electrons.
The elements which lie in the middle of the Group II A elements and the Group II B elements in the present day periodic table are the d block elements.
Electronic configuration of few elements is given below.
Question47 Write the atomic number of these elements.
For example all the elements of group 2 have an electron configuration of E ns 2 where E is an inert gas configuration and have notable similarities in their chemical properties.
Helium 1s 2.
The element misses out on the octet configuration by one electron.
Electronic Configuration of Group 1 and Group 2 Elements.

Eadie S Periodic Electron Configuration 2014 Chemistry Education Chemistry Lessons Teaching Chemistry

Alkaline Earth Metals Periodic Table Android App Element Chemistry Alkaline Earth Metals Electron Configuration

Equilibrium Class 11 Notes Chemistry Chapter 7 Learn Cbse In 2021 Chemistry Notes 11th Chemistry Solubility

Periodic Table Of Elements Edible 2d Fondant Birthday Etsy In 2021 Periodic Table Of The Elements Periodic Table Chemistry Periodic Table

Excellent Short And Informative What Are Periods And Groups In The Periodic Table Chemistry The Fus Science Websites Periodic Table Chemistry

The Electronic Configuration Is The Distribution Of Electrons Of An Atom Or Molecule In Its Orbital L Electron Configuration Graphing Quadratics Learn Biology

Tetryonics 53 26 Iron Is The Most Common Element By Mass Forming Our Planet Iron Metal Has Transition Metal Electron Configuration Alkaline Earth Metals

The S Block Elements Cbse Notes For Class 11 Chemistry Learn Cbse 11thchemistrynotes Thes Blockeleme Chemistry Notes 11th Chemistry Notes 11th Chemistry

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

Answer Key To The Periodic Table Scavenger Hunt Worksheet Related Teaching Chemistry Science Worksheets Teaching Middle School Science

What Are The First 20 Elements Periodic Table Of The Elements Periodic Table Words Periodic Table Art

Internet Database Of Periodic Tables Chemogenesis Electron Configuration Periodic Table Group Theory

This Shows Some Of The Elements Ionic Radius Potassium Is K The Ionic Radius Is 133 Ionic Radius Teaching Science Element Project

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

Structure Of Atom Class 11 Notes Chemistry Chapter 2 Discovery Of Electron Discharge Tube Experiment In 1 In 2021 11th Chemistry Notes 11th Chemistry Chemistry Notes

Image Of Page 25 A Comprehensive Treatise On Atomic And Molecular Structure By C H Cecil Henry Douglas Clark Publishe Molecular Structure Molecular Atom









No comments:
Post a Comment