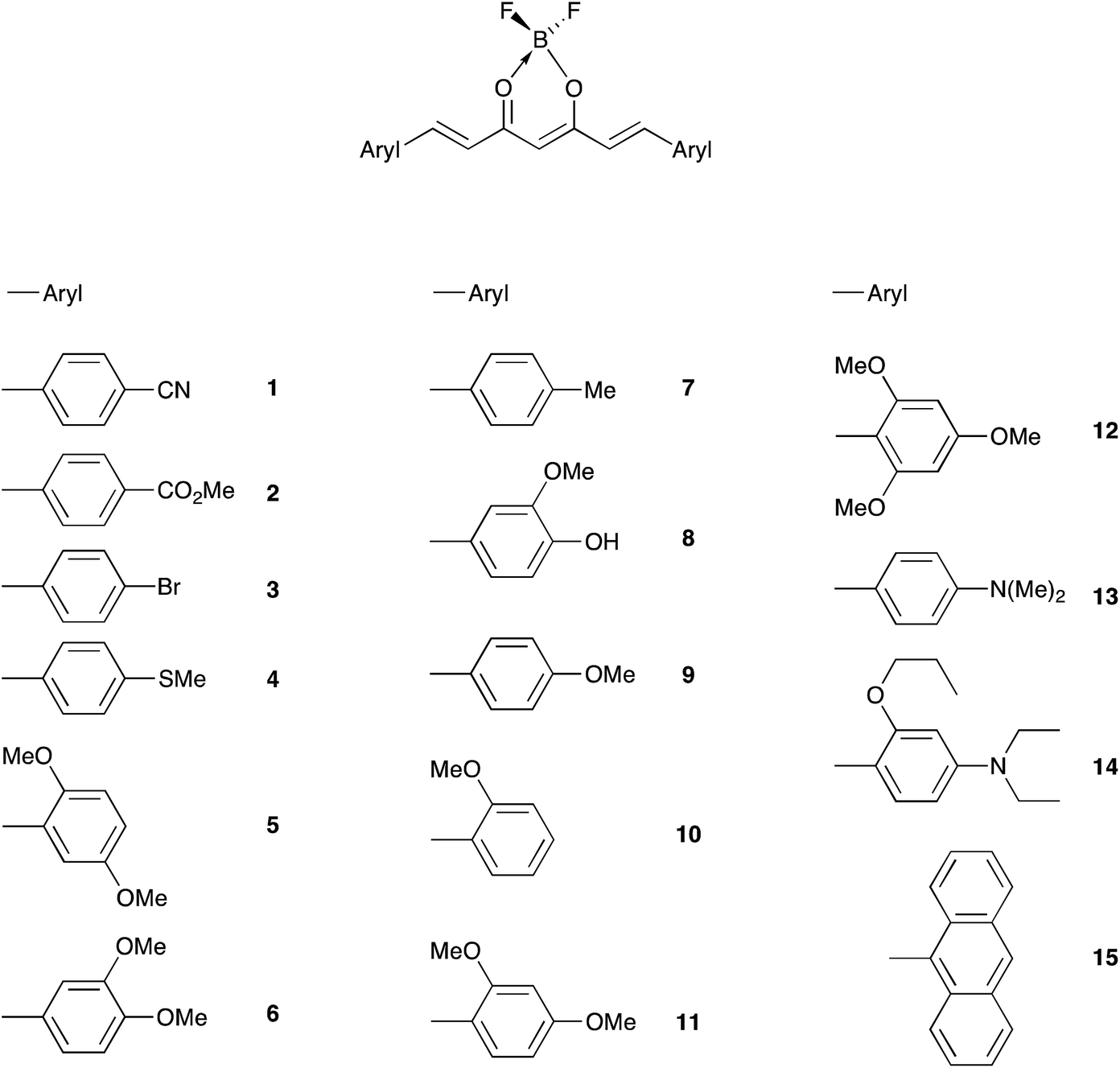
Influence Of The Electron Donor Groups On The Optical And Electrochemical Properties Of Borondifluoride Complexes Of Curcuminoid Derivatives A Joint Rsc Advances Rsc Publishing Doi 10 1039 C6ra25436e


In Cl No2 F Br I C2h5 And Och3 What Is Their Arrangement From The Highest Or Most Acidic Regarding Their I Effect Quora
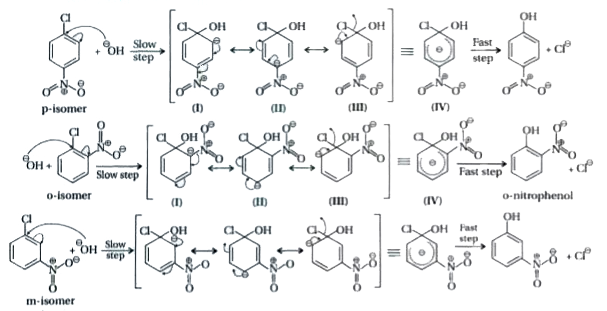
Explain Why The Electron Withdrawing Groups Such As No 2 Show Its Effect Only At O Position And P Position And Not At M Positions
Is It True That Fluorine Is Always The Strongest Electron Withdrawing Group Ewg Due To Fluorine S Unrivaled Electronegativity Quora

Influence Of The Electron Donor Groups On The Optical And Electrochemical Properties Of Borondifluoride Complexes Of Curcuminoid Derivatives A Joint Rsc Advances Rsc Publishing Doi 10 1039 C6ra25436e
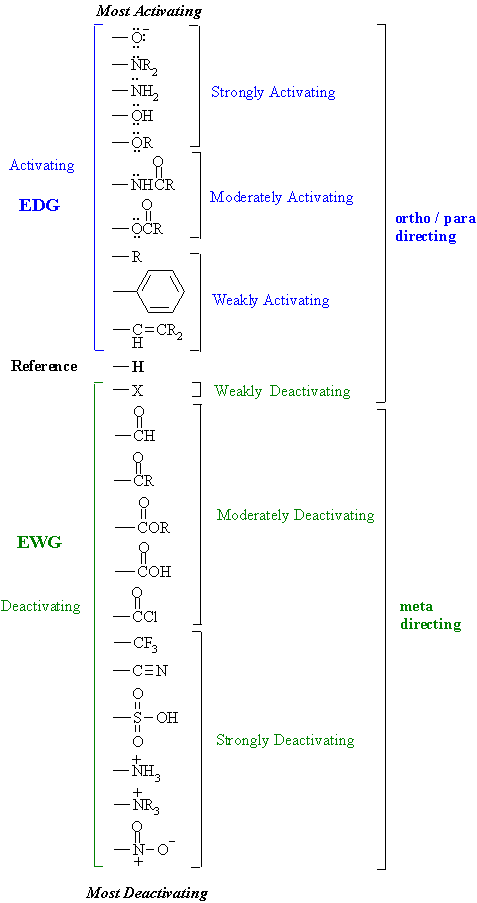

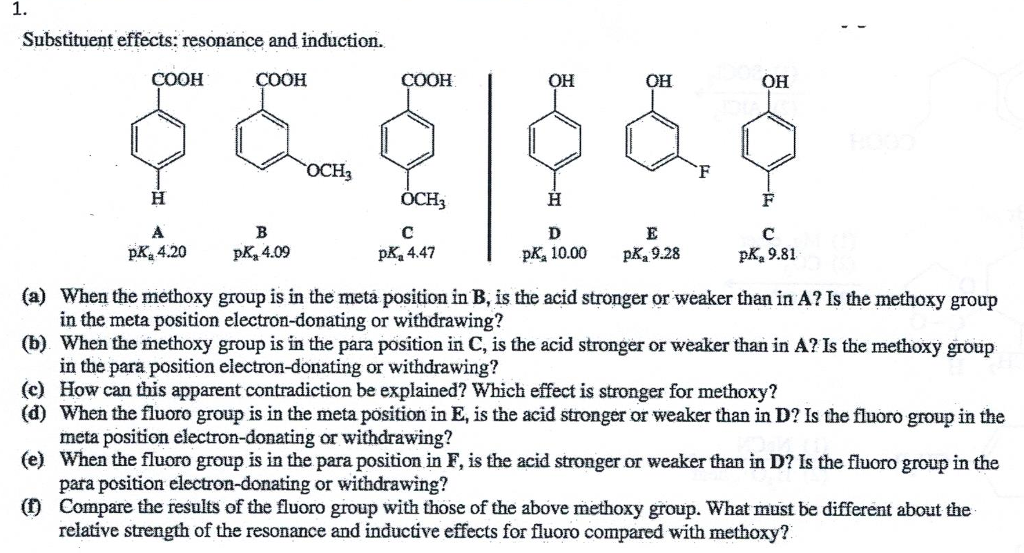







No comments:
Post a Comment