Lastly there is a single unpaired electron on the nitrogen atom. Below is the electron dot structure for a Nitrogen molecule.
Thus as per the electronic configuration of the element ie.

Electron dot structure nitrogen.
The atomic number is also equal to the number of electrons present in a neutral atom of an element.
The true electron configuration of nitrogen dioxide is considered to be some average of the two resonance structures given above.
413 Lewis Representation of Simple Molecules the Lewis Structures The Lewis dot structures pr ovide a pictur e.
A hybrid quantum system including a carbon nanotube double quan-tum dot and two nitrogen-vacancyNV centers is proposed for the generation of entanglement 20.
Use the element name mass and charge to determine the number of protons neutrons and electrons.
Molecules are made up of atoms that are stuck together in a particular shape or formNot all combinations of atoms are equally possible.
Now show that a metal-free hybrid material composed of carbon and nitrogen can promote this reaction all on its own with the help of some visible light.
The atomic number of lithium is 3 meaning that a neutral lithium atom has 3 protons and therefore also has 3 electrons.
This free radical explains the majority of nitric oxides chemical behavior.
Because of this the 4s orbital is filled before the 3d orbital.
If a molecule were split into smaller pieces it would be a different substance.
Atoms make certain shapes in preference to others.
In a manner analogous to the above free radicals are formed by the breaking of the nitrogennitrogen bond in aromatic hydrazines of the general structure R 2 NNR 2 or of the central nitrogennitrogen bond in aromatic tetrazanes R 2 NRNNRNR 2.
Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule.
The Lewis structure of nitrogen dioxide is also interesting because there is a single unpaired valence electron on the central nitrogen atom.
The lung can be exposed to a variety of reactive nitrogen intermediates through the inhalation of environmental oxidants and those produced during inflammation.
In this structure a carbon nan-otube bridges from one reservoir to another one and allows electrons to be confined by applying gate voltage.
In fact this structure is misleading because it suggests that the two oxygen atoms on the right-hand side of.
A Lewis structure also called Lewis dot formulas Lewis dot structures or electron dot structures are pictorial diagrams that represent the bonding between atoms in a.
Classically known as a major component of both indoor and outdoor air pollution NO2 is a toxic free radical gas.
A molecule is the smallest amount of a chemical substance that can exist.
The above image shows the lewis Structure of single nitrogen and a hydrogen atom.
S p d f.
For many molecules the sharing of electrons allows each atom to attain the equivalent of a full valence.
It is a stable hydride formed of one nitrogen and three hydrogen atoms.
The only exception to these rules is the 3d orbital which has slightly higher energy than the 4s orbital.
Found that a narrow-line tunable laser combined with a scanning tunneling microscope was able to generate photoluminescence spectra of the electronic and vibrational states of single molecules with microelectron.
In the Periodic Table Nitrogen is placed in Group 5 across Period 2.
CHEMICAL BONDING AND MOLECULAR STRUCTURE 103 When combining atoms share three electron pairs as in the case of two nitrogen atoms in the N 2 molecule and the two carbon atoms in the ethyne molecule a triple bond is formed.
N 4 is higher in energy than n 2 The subshells increase in energy as follows.
As the p shell needs to accommodate a maximum of six electrons there is a scarcity of three electrons.
Hence the formula of NF3 becomes AX 3 N 1 So according to the VSEPR chart if the molecule has the formula of AX 3 N 1 it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral.
As per the molecule N2 it has two atoms of Nitrogen.
One of these regions however is a lone pair which is not included in the molecular structure and this lone pair influences the shape of the molecule Figure 5.
Predict how addition or subtraction of a proton neutron or electron will change the element the charge and the mass.
Thus the radical 11-diphenyl-2-picrylhydrazyl structure II exists as a stable.
The principal quantum shells increase in energy with increasing principal quantum number.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
One of the oxygen atoms can be thought of as attaching to the nitrogen via a coordinate bond using the lone pair on the nitrogen atom.
It can form an NH4 ion by accepting a proton.
Define proton neutron electron atom and ion.
Reactive nitrogen species RNS include nitrogen dioxide NO2 and peroxynitrite ONOO-.
The atomic number of the nitrogen is seven which makes its electronic configuration 1s2 2s2 2p3.
So nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the lewis dot structure of NF3.
The molecule has a pungent smell.
The atomic number or proton number is the number of protons in the nucleus of an atom and has the symbol Z.
The photocatalyst combines one material C3N4 known to split water into hydrogen and peroxide with a.
The electron configuration of aluminum is.
Splitting water into its constituent elements hydrogen and oxygen generally requires the assistance of metal catalysts.
A Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds.
This procedure provides constructing a DQD.
Lewis dot diagram for AlCl_3 AlCl 3 like BF 3.
It makes a single nitrogen atom to have five valence electrons.
On the other hand the ammonia molecule NH 3 also has four electron pairs associated with the nitrogen atom and thus has a tetrahedral electron-pair geometry.
Microscopic understanding and molecular-level control of individual electronic quantum states of a single molecule are a long-standing challenge in spectroscopy.
In this blog post we will learn about the Lewis dot structure electron geometry and molecular geometry of this molecule.
25 it has five electrons in its outermost valence shell.
Steps to Draw the Lewis structure of N2.

12 Mg Magnesium Electron Shell Structure Schoolmykids Electron Configuration Element Chemistry Magnesium

Electron Configuration For Pb Pb2 And Pb3 Lead And Lead Ions Electron Configuration Electrons Configuration

Im Dunkeln Leuchten Lewis Dot Diagramme Senior Chemie Chemie Chemie D Chemie Dot Organic Chemistry Study Teaching Chemistry Chemistry Lessons
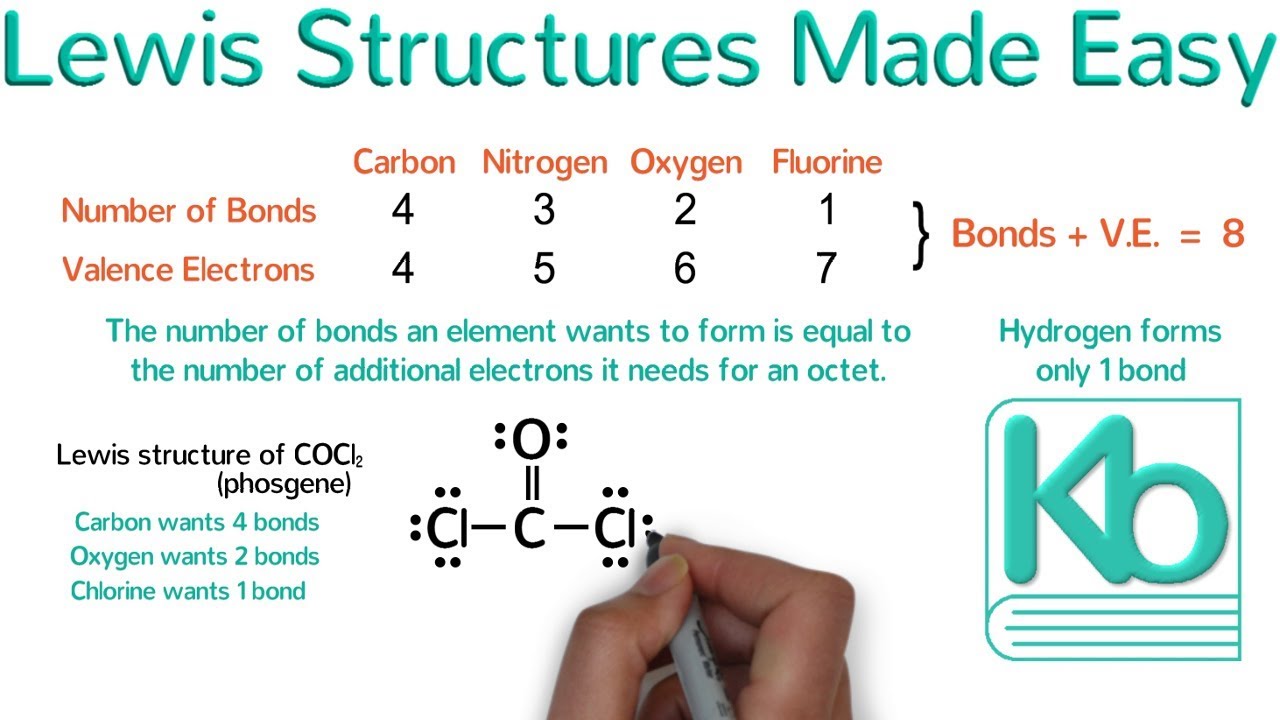
Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo High School Chemistry Teaching Science Organic Chemistry Study

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

Lewis Dot Structure For Hydrogen H Lewi Electron Configuration Chemistry Worksheets Chemical Equation

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atomic Structure Atom Diagram

Bohr Model Worksheet Answers Fresh Drawing Bohr Models Worksheet Chemistry Worksheets Bohr Model Atomic Structure

See The Electron Configuration Of Atoms Of The Elements Neon Atom Electron Shell Diagram Atom Diagram Electron Configuration Neon Atom

















No comments:
Post a Comment