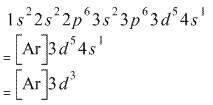

Give The Electronic Configuration Of Mn2 And Cr3 Chemistry Some Basic Concepts Of Chemistry 13094403 Meritnation Com

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2

13 Write The Complete Electron Configuration And Indicate The Number Of Unpaired Electrons For Each Of The Followin Homeworklib
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcq Xyy60dwyv6oi9imyjvwvc43r3bysn0d1gyln4xio3w1vhg4x Usqp Cau
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrpcqkgihonwucw1hkq F03kohphgesenbmbdgd5zwklbp1agoa Usqp Cau

Write Down Electronic Configuration Of Cr3 And Cu2 Ions Calculate The Number Of Unpaired Electrons Brainly In
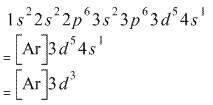
How Is The Electron Configuration Of Cr3 1s2 2s2 2 6 3s2 3p6 3d5 4s1 Ar 3d5 4s1 Ar 3d3 Home Work Help Learn Cbse Forum

Give The Electronic Configuration Of Mn2 And Cr3 Chemistry Some Basic Concepts Of Chemistry 13094403 Meritnation Com

What Is The Configuration Of Chromium 3 According To Crystal Field Theory In Aqueous Medium Chemistry 13544091 Meritnation Com

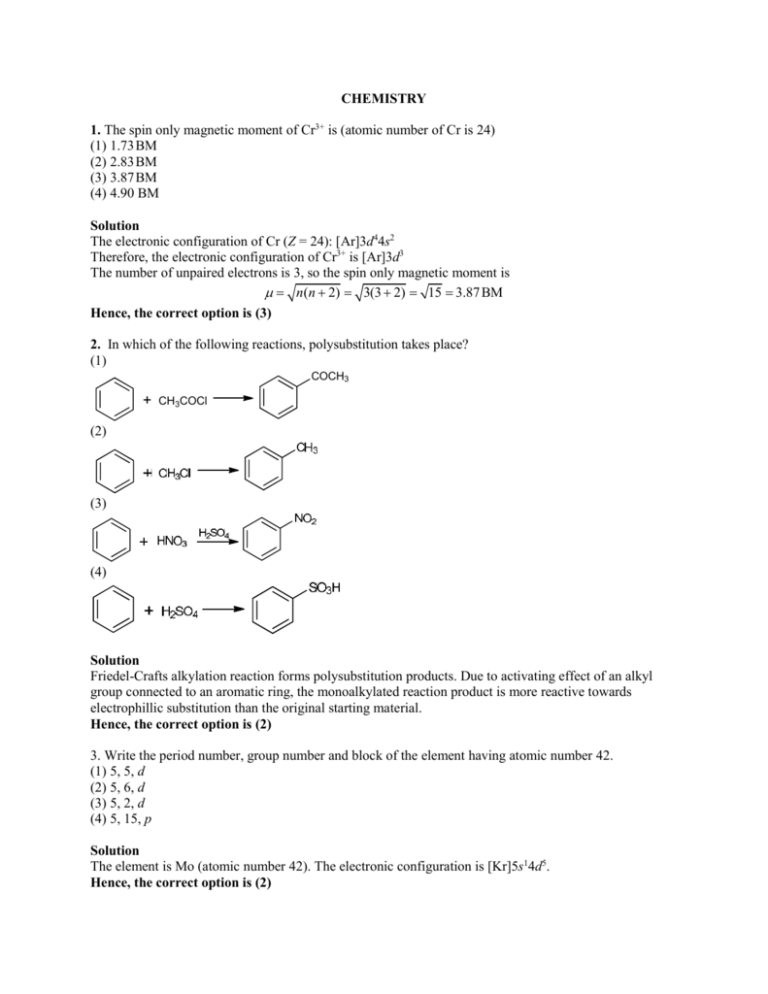
The D Electron Configurations Of Cr3 Mn2 Fe2 And Co2 Are D4 D5 D6 And D7 Respectively Which One Of The Following Will Exhibit Minimum Paramagnetic Behaviour At No Cr 24














No comments:
Post a Comment